This month, we shared some festive content on our social media relating to our three themes: Additive Manufacturing, Chemicals & Hydrogen, and Surfaces & Coatings! We have decided to share them here, so you can discover the science behind Christmas in one place.
Day 1: Chemistry of snow

- Ice is less dense than water. The fixed hydrogen bonds in ice hold water molecules further apart than in its liquid state.
- The hexagonal structure of snowflakes is due to water molecules forming hydrogen bonds to four other molecules.
- The shape of a snowflake depends on humidity and temperature during formation. 121 categories of snowflakes have been identified.
Day 2: History of tinsel

- Tinsel used to be expensive: in the 19th century it was made from thin strips of tin-laminated sheet brass and silver-laminated copper wire.
- In the 20th century, mass production of tinsel met with a demand for copper during WWI, with aluminium tinsel becoming a popular variety,
- Lead alloy was also a popular component of tinsel until the 1960s, when the risks of lead poisoning become well known.
- Tinsel gets its shiny finish by heating and evaporating metal such as aluminium under a vacuum and condensing it onto the plastic to leave a thin coating.
Day 3: History of the bauble

- Glass baubles used to be very popular; first made in Germany in the mid-19th century, they were exclusively produced in Germany until 1925.
- Baubles are now mostly made from plastic, decorated with metallic coloured spray.
- Unique and personalised 3D printed baubles are rising in popularity, with many small businesses offering bespoke bauble designs.
Day 4: Chemistry of fake snow

- Fake snow may be made from sodium polyacrylate, a super-absorbent polymer.
- This same polymer is used in applications such as wound dressings and nappies.
- When wet, the molecules in the polymer become negatively charged carboxylate ions and sodium ions, which repel each other, causing expansion.
Day 5: Fibre optic trees
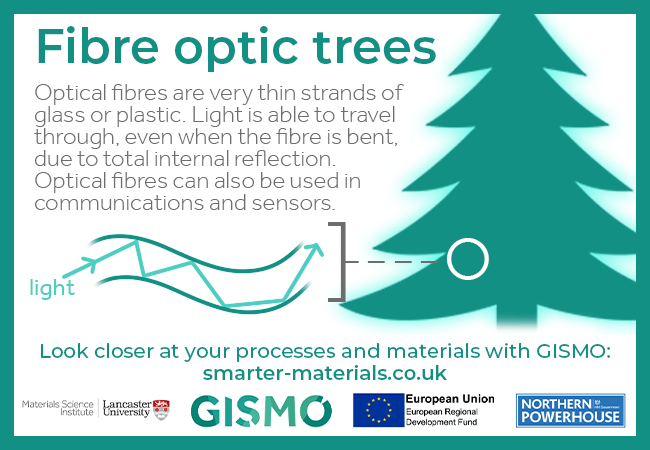
Optical fibres are very thin strands of glass or plastic. Light is able to travel through, even when the fibre is bent, due to total internal reflection. Optical fibres can also be used in communications and sensors.
Day 6: What’s in mulled wine?
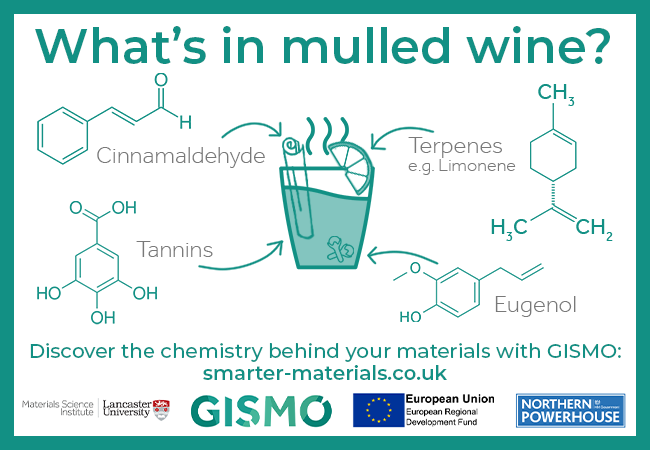
What’s in mulled wine? A few of the ingredients include cinnamaldehyde, terpenes such as limonene, tannins, and eugenol.
Day 7: Chemistry of cinnamon

- Cinnamaldehyde is an oily yellow liquid that give cinnamon its signature taste and smell.
- It can be made synthetically, but is commonly obtained from the steam distillation of the oil of cinnamon bark, which is a much more efficient process.
- Eugenol can be extracted from cinnamon essential oil, and has antiseptic and analgesic properties.
Day 8: Cooking the perfect turkey
Rubbing the turkey with salt 24 – 48 hours before cooking allows osmosis to balance the salt content in the turkey, while diffusion allows salt to travel to areas with less salt content.
Once evenly distributed, salt loosens the meat’s protein strands, locking in water during roasting.
This also allows water to be drawn away from the skin, making it crispier.
As well as osmosis and diffusion, the Maillard reaction gives turkey its distinctive flavour.
Day 9: Science of Christmas lights
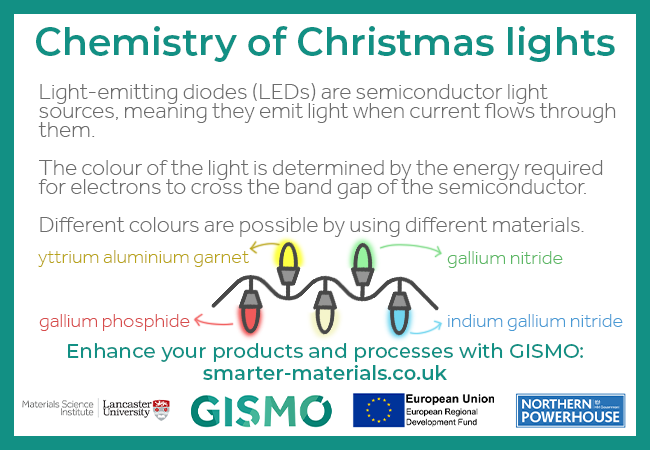
Light-emitting diodes (LEDs) are semiconductor light sources, meaning they emit light when current flows through them.
The colour of the light is determined by the energy required for electrons to cross the band gap of the semiconductor.
Different colours are possibly by using different materials. For example, gallium phosphide for red, yttrium aluminium garnet for yellow, gallium nitride for green, and indium gallium nitride for blue.
White LEDs use blue LEDs with phosphor coatings, which yellow light through fluorescence. The combination of yellow and blue light appears white to the eye.
Day 10: What makes crackers pop?
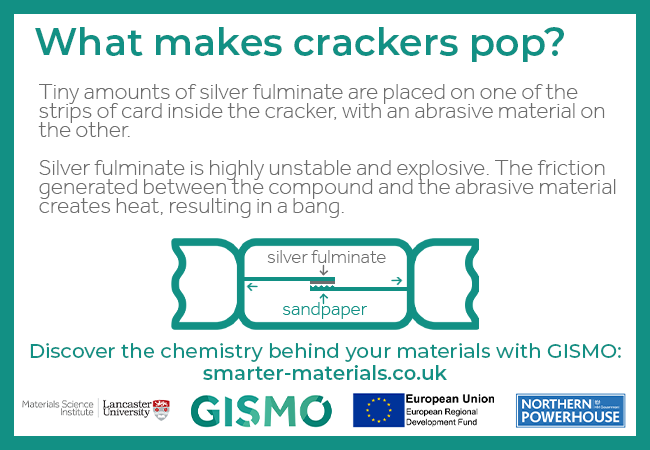
Tiny amounts of silver fulminate are placed on one of the strips of card inside the cracker, with an abrasive material on the other.
Silver fulminate is highly unstable and explosive. The friction generated between the compound and the abrasive material creates heat, resulting in a bang.
Silver fulminate is the highly explosive silver salt of fulminic acid. It is used in very small quantities in novelty explosives such as crackers, but in too large a quantity it can self-detonate under its own weight
Day 11: Building the perfect snowman

- Ideal temperature is 1 degrees Celsius, allowing snow to be suitably dense and moist.
- Add more water if snow is dry.
- Build on stable, shady grass.
- Build in 3 balls for slower melting.
- Build the base, middle and head in a ratio of 3:2:1.
- Pack in extra snow in between balls to secure.
Day 12: How do candles burn?
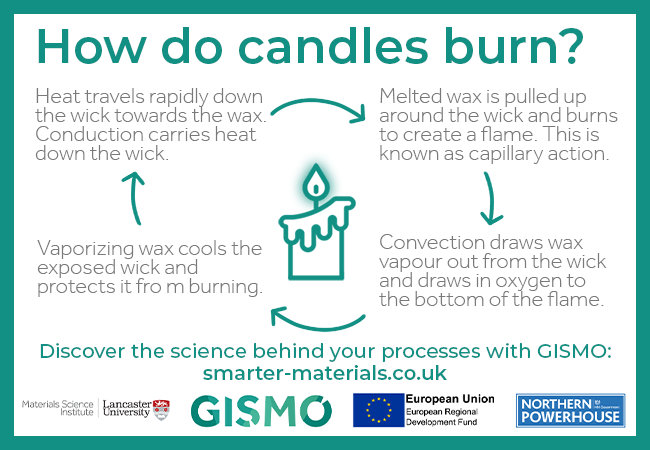
- Heat travels rapidly down the wick towards the wax. Conduction carries heat down the wick.
- Melted wax is pulled up around the wick and burns to create a flame. This is known as capillary action.
- Convection draws wax vapour out from the wick and draws in oxygen to the bottom of the flame.
- Vaporizing wax cools the exposed wick and protects it from burning


